Abstract
Introduction: Chimeric antigen receptor modified T cells directed against CD19 (CART19) has demonstrated efficacy in relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) with durable complete remissions (CR) [Schuster et al. Blood 2016 (abstract 3026); Kochenderfer et al. Blood 2010; Turtle et al. Sci Transl Med 2016 ]. However, non-responding patients require novel therapeutic approaches. Pembrolizumab, an anti-PD1 checkpoint inhibitor, is an attractive next therapeutic option given its T cell-mediated mechanism of action. Anecdotally, we observed responses in patients treated with PD1 blockade after CART19 (Chong et al. Blood 2017). Based on these observations, we are conducting a clinical trial of pembrolizumab in patients with B cell non-Hodgkin lymphomas expressing CD19 who are r/r after CART19.
Methods: This prospective single-institution trial enrolled patients with progressive B-NHL after treatment with CART19 expressing either a murine (CTL019) or a human (CTL119) CD19-specific single chain antibody fragment fused in tandem with 4-1BB and TCRζ costimulatory / activation domains (NCT02650999). Patients who received intervening therapy between CTL019/119 infusion and enrollment were excluded. Patients received a fixed dose of pembrolizumab 200mg IV every 3 weeks until progression of disease, therapy limiting-toxicity, or elective protocol discontinuation. The primary endpoint was safety. Secondary endpoints included overall response rate (ORR) and progression-free survival (PFS) from initiation of pembrolizumab. Whole blood and peripheral blood mononuclear cells (PBMC) were collected from subjects at protocol-specified times for correlative studies. DNA extracted from whole blood was analyzed for the appropriate CAR transgene copy number using qPCR (sensitivity 25 copies/μg DNA). Flow cytometry for immunophenotyping was performed on single cell suspensions prepared from PBMC.
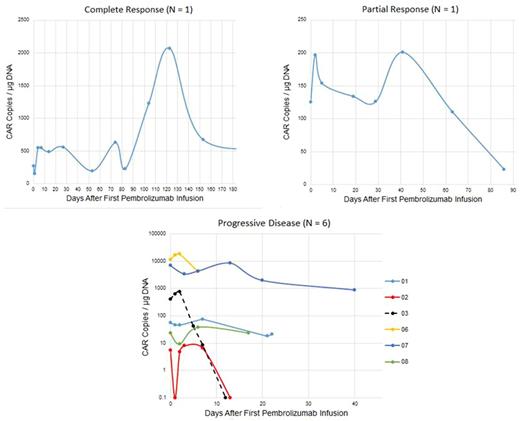
Results: From June 1, 2016 to June 1, 2017, 8 patients with DLBCL were enrolled [3 germinal center (2 "double hit"), 3 non-germinal center and 1 T-cell rich DLBCL, and 1 transformed follicular lymphoma). Median age was 56.7 years. Median prior therapies was 5 (range 3-7). Median PFS after CTL019/119 was 2.2 months (range 0.4-3.2); median time to first pembrolizumab dose was 2.5 months (range 0.4-3.7). Adverse events (CTCAE 4.0) per patient at least possibly related to pembrolizumab were: neutropenia (grade 4; N=3), cytokine release syndrome (grade 3 on Penn scale; N=1), delirium (grade 3; N=1), , infusion reaction (grade 2; N=1), fever (grade 1 or 2; N=3), dyspnea (grade 1; N=1), pleural effusion (grade 1, N=1), and arthralgia (grade 1; N=1). One patient developed CMV infection (unrelated; grade 4) resulting in discontinuation of protocol therapy. ORR after pembrolizumab was 25% [1 complete response (CR); 1 partial response (PR)]; 6 patients had progressive disease (PD). One patient with DLBCL continues in CR at 280 days; one patient with T-cell rich DLBCL in PR was removed from study because of CMV pneumonitis. Immunohistochemistry for PD-1 expression in the tumor microenvironment was performed for 5 patients; 4 patients had detectable PD-1 expression, including one patient with PR and 3 patients with PD. All patients, responders and non-responders, had a single initial peak in CAR transgene copy number after the first pembrolizumab dose with fold increases of 1.2-2.0 (median 1.6) between days 2-13 (median 2.5 days). Maximum CAR transgene copy number during therapy did not correlate with response, nor did the AUC over days 1-7; however, responding patients had more than one peak during therapy while non-responding patients had a single peak following the initial dose of pembrolizumab. Flow cytometry showed increases in both %CD4 CAR T cells and %CD8 CAR T cells in both responding and nonresponding patients.
Conclusions: PD1 blockade with pembrolizumab appears safe and results in clinical responses in some patients with progression of DLBCL after anti-CD19 directed chimeric antigen receptor modified T cell therapy. Analysis of the pharmacokinetics of CAR T-cells in patients treated with pembrolizumab appears to identify responding patients, and supports the hypothesis that CAR T cells may be rejuvenated by infusion of PD1 antagonists. Additional immunophenotypic and epigenetic studies are in progress to distinguish the mechanism of action in responding patients.
Melenhorst: Novartis: Research Funding. Svoboda: Kite: Consultancy; BMS: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene: Research Funding. Dwivedy Nasta: Incyte: Research Funding; Takeda: Research Funding; Immunogen: Research Funding. Landsburg: Curis: Consultancy, Research Funding; Takeda: Research Funding. Mato: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy, Research Funding; Kite: Consultancy; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy; DTRM: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding; Portola: Research Funding. Lacey: Novartis: Research Funding; Genentech: Honoraria. June: Novartis: Patents & Royalties, Research Funding; WIRB/Copernicus Group: Honoraria, Membership on an entity's Board of Directors or advisory committees; Tmunity Therapeutics: Equity Ownership, Research Funding; Celldex: Honoraria, Membership on an entity's Board of Directors or advisory committees; Immune Design: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Schuster: Seattle Genetics: Consultancy; Merck: Research Funding; Nordic Nanovector: Consultancy; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal